Seeking to restore women’s independence from OAB with bioelectronic medicine
 Mechanism of Action
Mechanism of Action
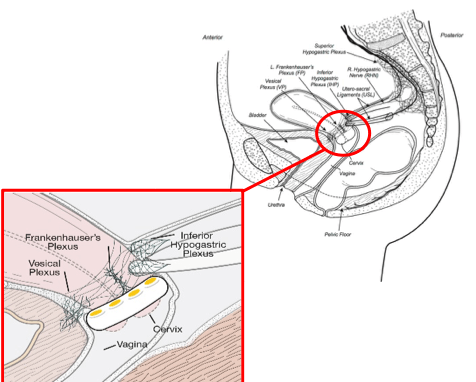
Targeting the “Gateway”
FemPulse technology is intended to target nerves that serve as the gateway between pelvic visceral organs and the central nervous system to restore neurological control of bladder function.
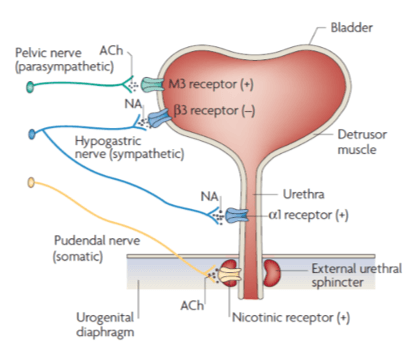
Innervation of the lower urinary tract
FemPulse therapeutic parameters were set with the intention of selectively targeting the sympathetic aspect of the autonomic nervous system involved in relaxation of the bladder and constriction of the urethral sphincter.
Clinical Studies in OAB
FemPulse will seek initial FDA 510(k) clearance as a nonimplanted electrical continence device
First-in-human clinical study complete
The first clinical study was a blinded, randomized, sham-controlled crossover study. Clinical results were presented at the American Urology Association (AUA) annual meeting in San Francisco and International Urogynecological Association (IUGA) annual meeting in Vienna, Austria. Dr. Suzette Sutherland, Director of Female Urology at University of Washington, presented that primary objectives regarding fit, comfort and safety were met, as well as positive secondary outcomes regarding patient preference, a reduction in incontinence events and an increase in time between voids.
More information about the trial is available at www.clinicaltrials.gov, identifier (NCT number): NCT03874637.
In-home study
The EVANESCE-OAB™ (Evaluation of a Non-Implanted Electrical Stimulation Continence Device for Overactive Bladder) trial is a prospective, randomized, sham-controlled, double-blind, parallel group design clinical investigation. The study enrolled 21 patients across three sites to assess in an ambulatory setting the treatment in adult women with a diagnosis of overactive. The primary objectives of the trial are to evaluate safety, describe fit and comfort and assess the potential clinical utility of FemPulse’s proprietary device therapy. The company expects results from the trial to be presented at medical meetings in 2020.
More information about the trial is available at www.clinicaltrials.gov, identifier (NCT number): NCT03784170.
Articles Featuring FemPulse
- MD+DI: Finally, a Wearable Neuromodulation Device for Overactive Bladder
- MedTech Insight: Neuromodulation Devices Stimulate Opportunities in Urology
- Start Up: FemPulse Pioneers On Multiple Fronts (article republished by MedTech Insight)

 Mechanism of Action
Mechanism of Action